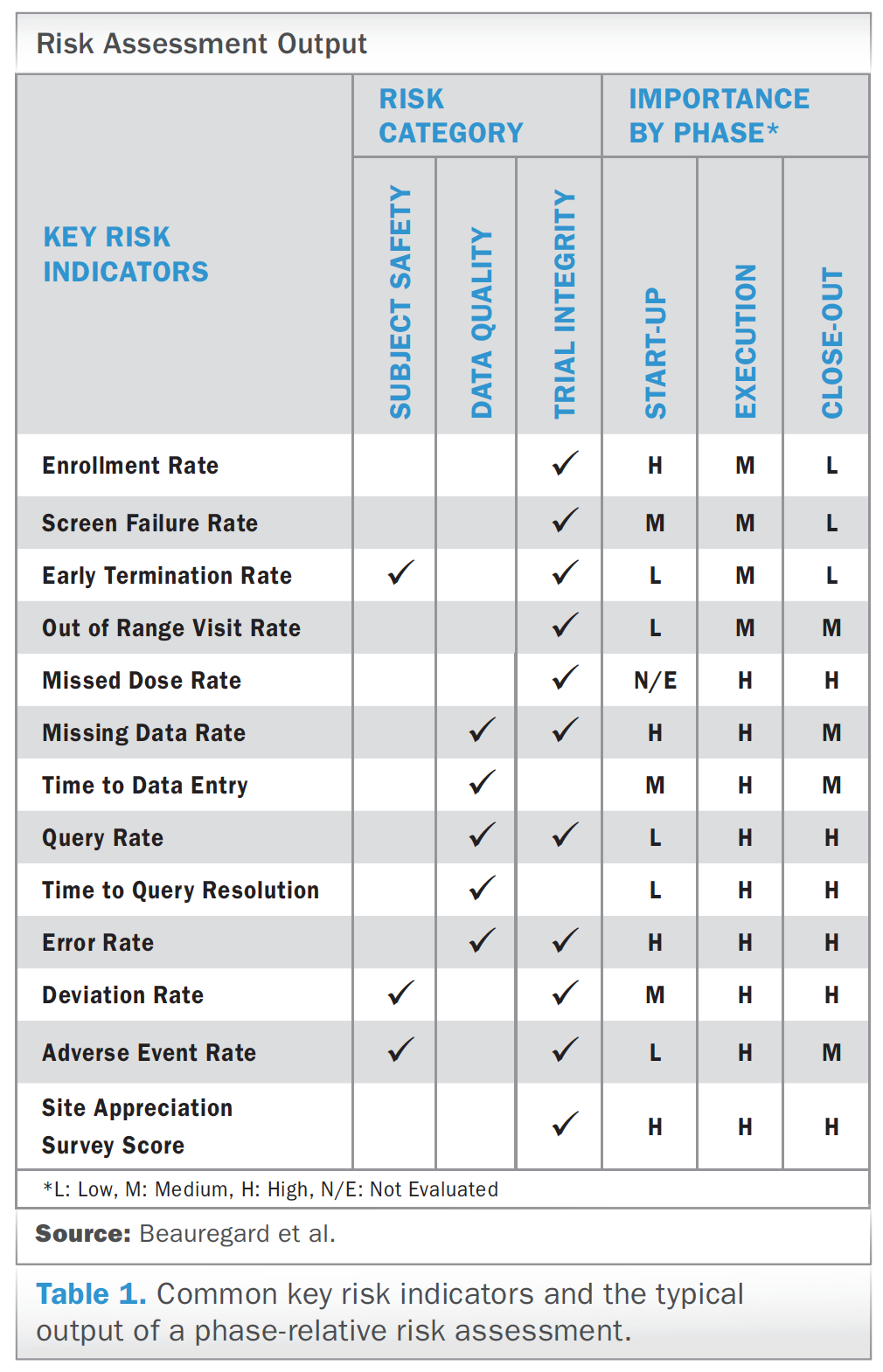
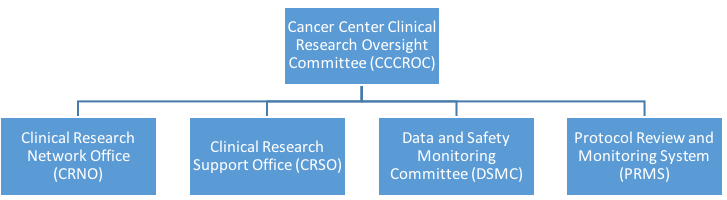
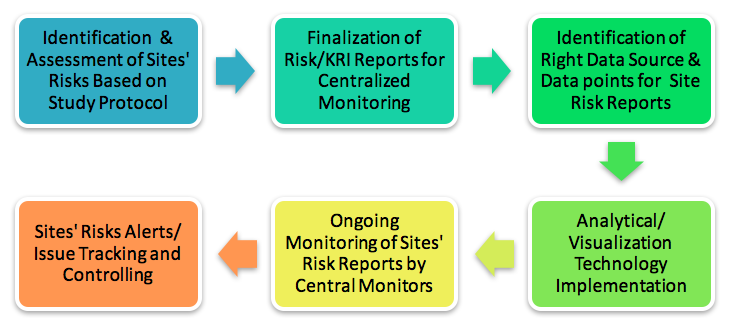
Risk Based Quality Management (RBQM) - A Collaborative Approach To Holistic Clinical Trial Oversight
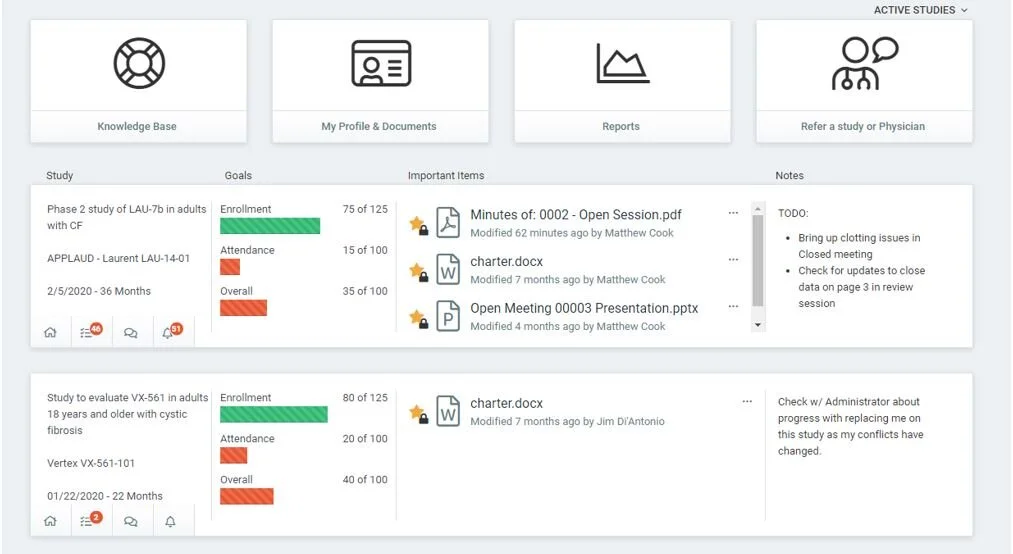
Clinical Trial Oversight | DSMB Software | Data Monitoring Committee in Clinical Trials | Cloud Concinnity®
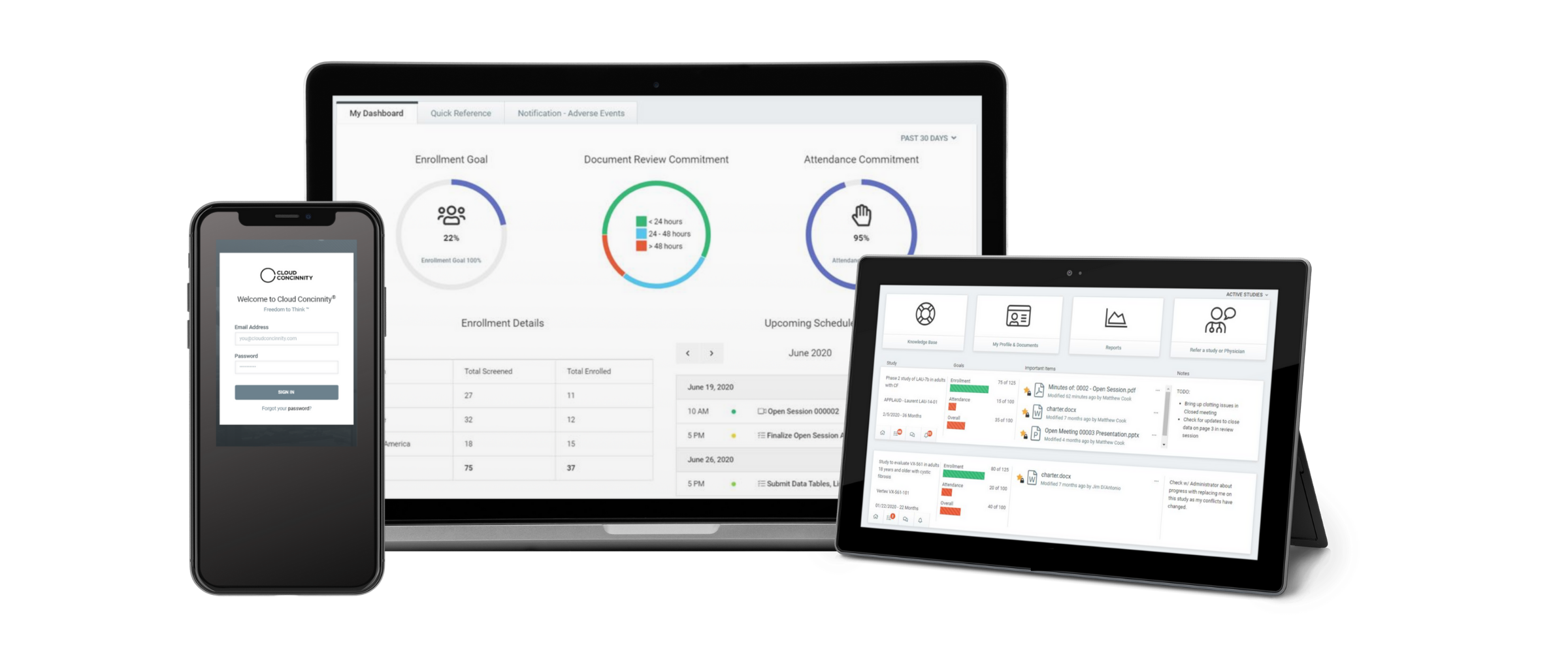
Clinical Trial Oversight | DSMB Software | Data Monitoring Committee in Clinical Trials | Cloud Concinnity®

Modernizing Clinical Trial Oversight: The Path to Clinical Operations Excellence | Medidata Solutions - Medidata Solutions

Operationalizing Good Clinical Practice Principles in Pediatric Clinical Research - The Journal of Pediatrics

Oversight or Overkill? – Managing a CRO Without Duplicating Everything They Do - Clinical Trials Arena

Post-Trial Responsibilities - The Multi-Regional Clinical Trials Center of Harvard and Brigham and Women's Hospital