Figure 1 from Knowledge-data integration for temporal reasoning in a clinical trial system | Semantic Scholar
Therapeutics for Dengue: Recommendations for Design and Conduct of Early-Phase Clinical Trials | PLOS Neglected Tropical Diseases

Effectiveness and safety of electroacupuncture for poststroke patients with shoulder pain: study protocol for a double-center, randomized, patient- and assessor-blinded, sham-controlled, parallel, clinical trial | Semantic Scholar

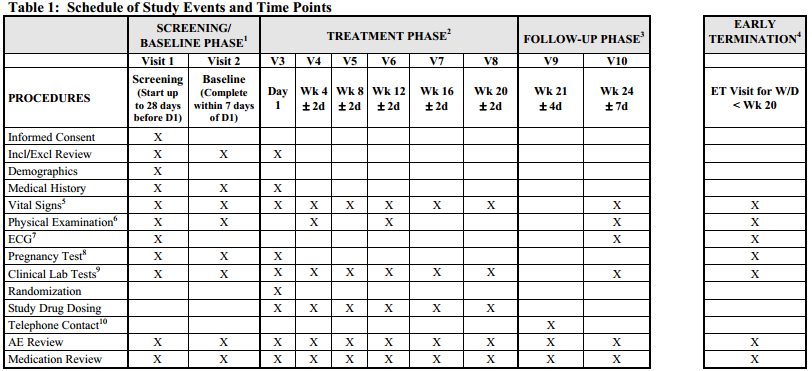
Schedule of Events. Example visit and assessment specification from a... | Download Scientific Diagram

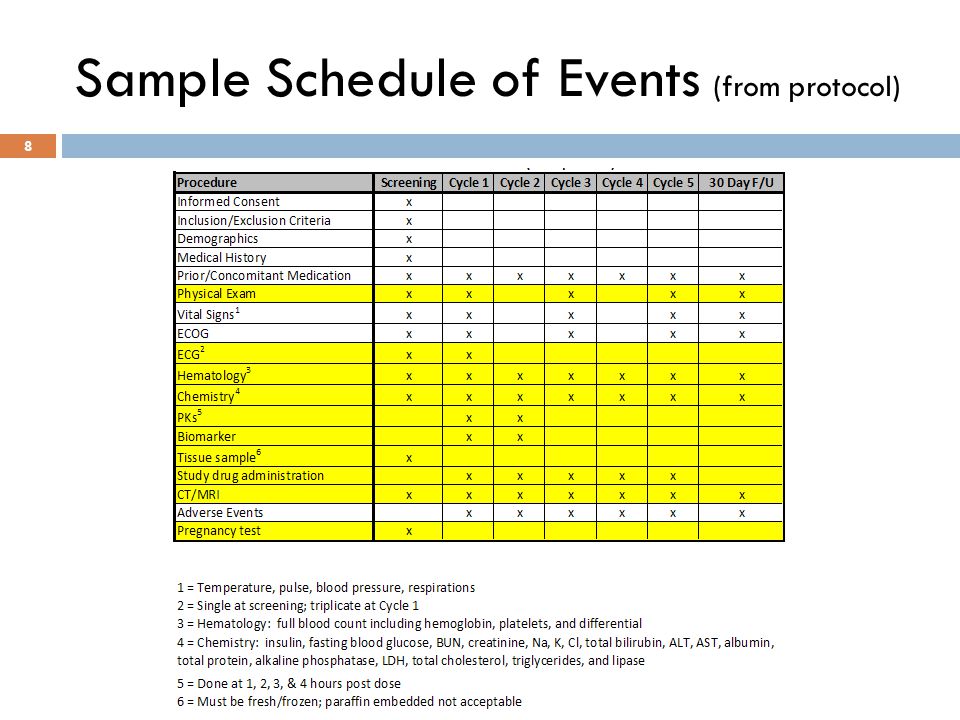
David Cloutier Director, Research Center Management and Development Budgeting for Industry Sponsored Clinical Trials. - ppt download

NHSBT/MRC Clinical Studies Unit Platelets for Neonatal Transfusion Study 2 (PlaNeT-2) A randomised controlled trial of platelet transfusion thresholds. - ppt download