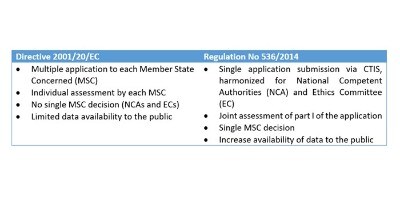
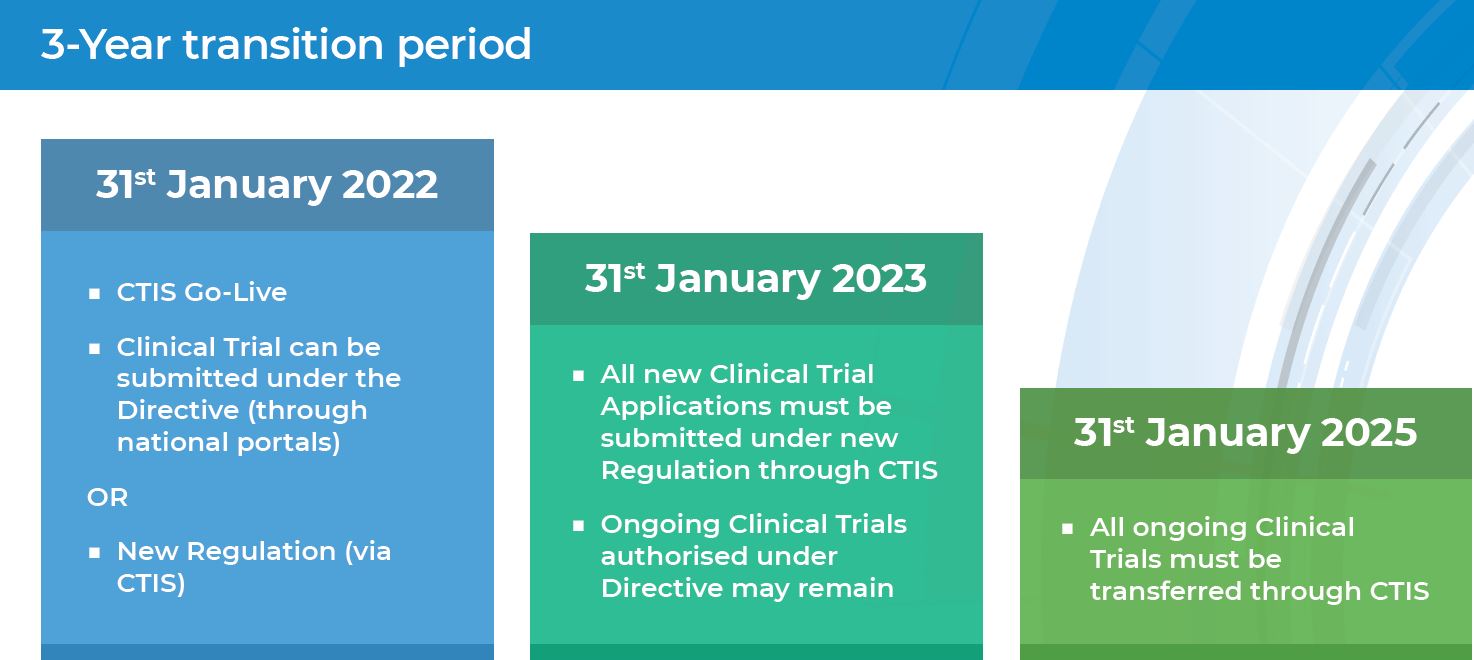
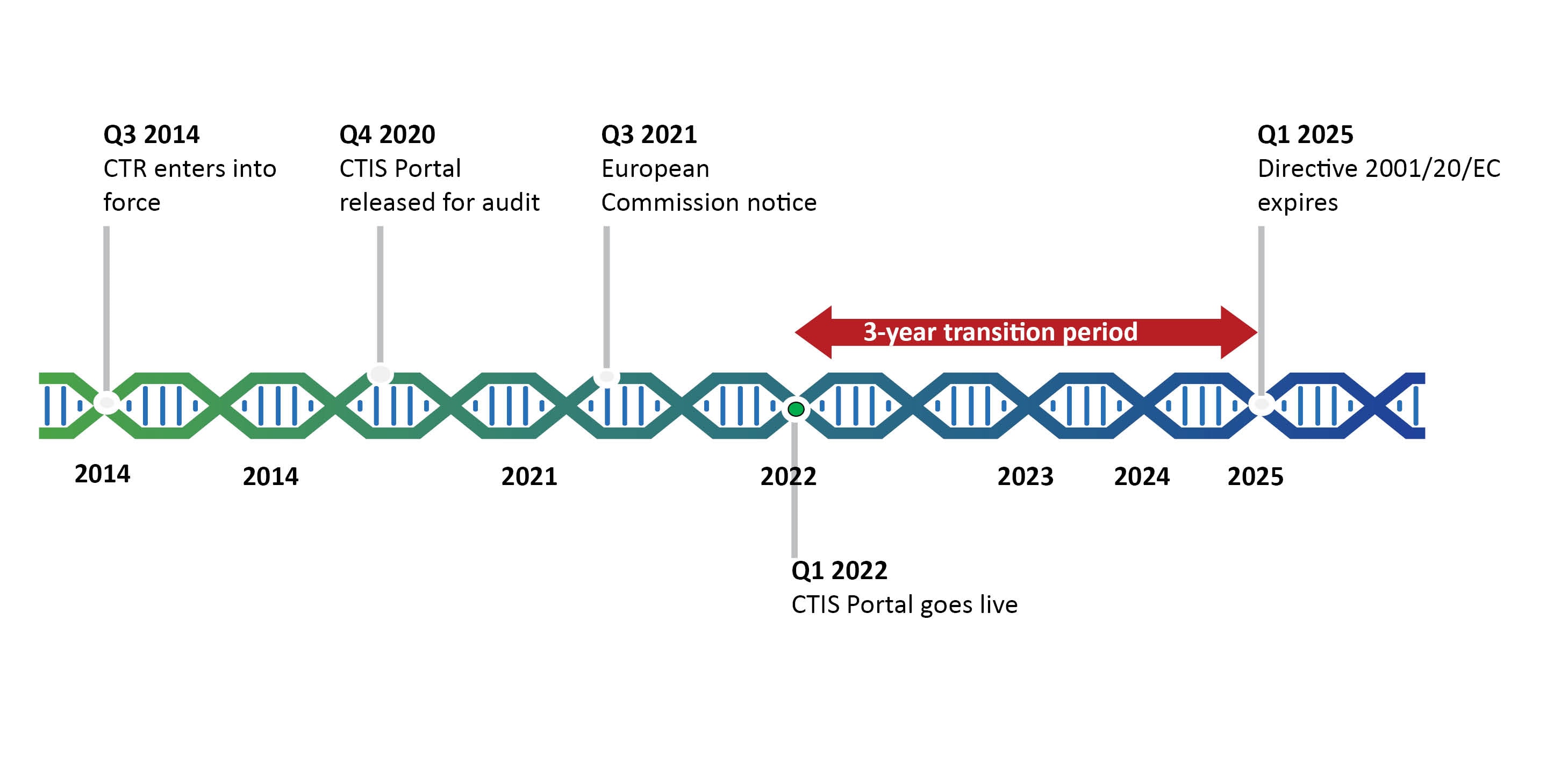
EORTC EU Clinical Trials Directives Organisation and Implementation of Cancer Clinical Trials Anastassia Negrouk EORTC Regulatory Affairs Manager Intergroup. - ppt download
The Clinical Trials Directive: How Is It Affecting Europe's Noncommercial Research | PLOS Clinical Trials
PLOS Clinical Trials: The Clinical Trials Directive: How Is It Affecting Europe's Noncommercial Research

European Clinical Trial Directive (Directive 2001/20/EC) dr. Cees Smit (NPCF/EGAN) EPF Annual Meeting May 19, Brussels. - ppt download

CT01: How to Gain and Maintain Approval for Clinical Research Under the EU Clinical Trials Directive | Zenosis – Learning for Life