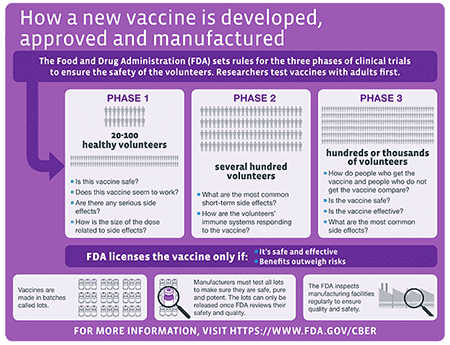
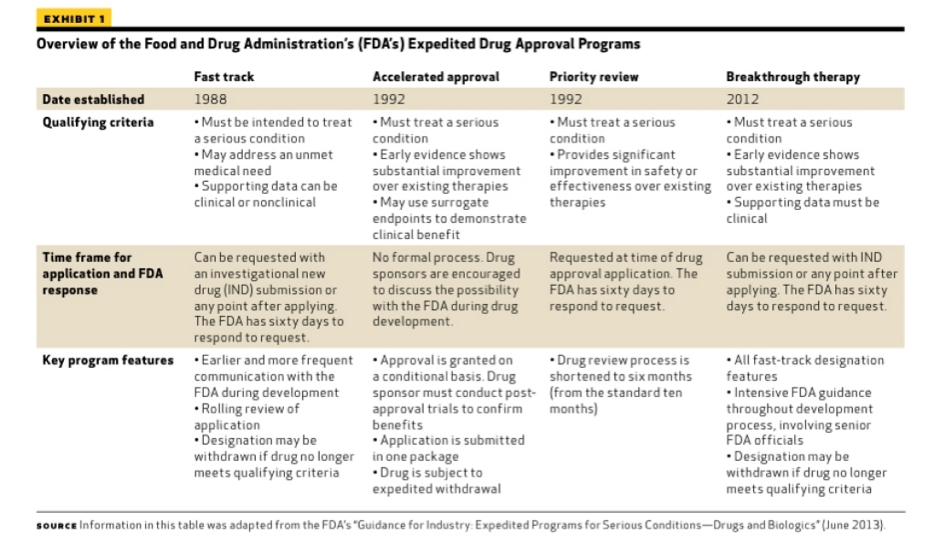
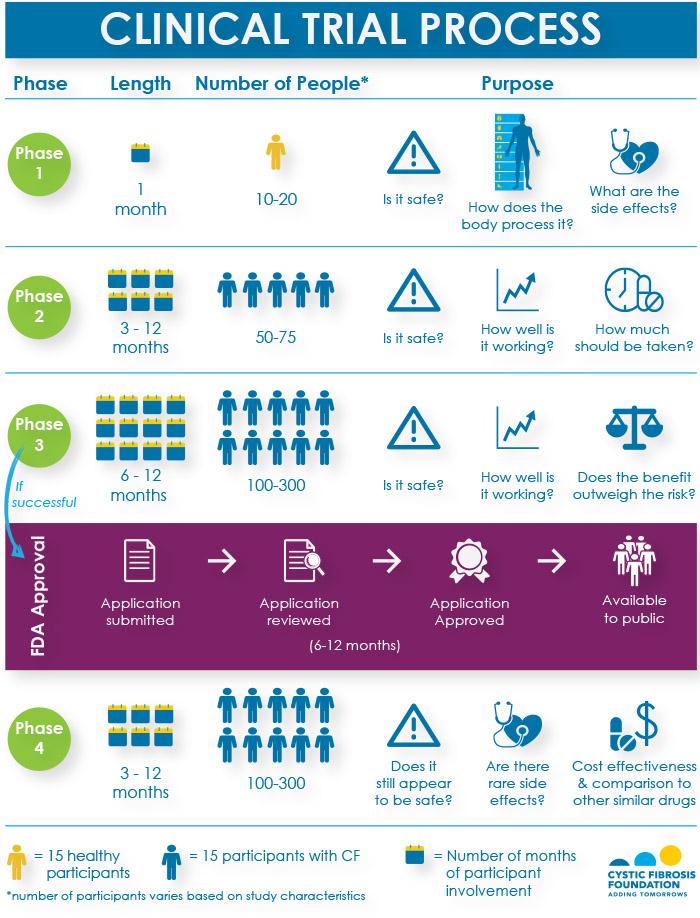
Roadmap to 2030 for Drug Evaluation in Older Adults - Liu - - Clinical Pharmacology & Therapeutics - Wiley Online Library

Book 13: 2021 FDA Guidance on Clinical Study Reports and Statistical P – Clinical Research Resources, LLC

Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study - The Lancet