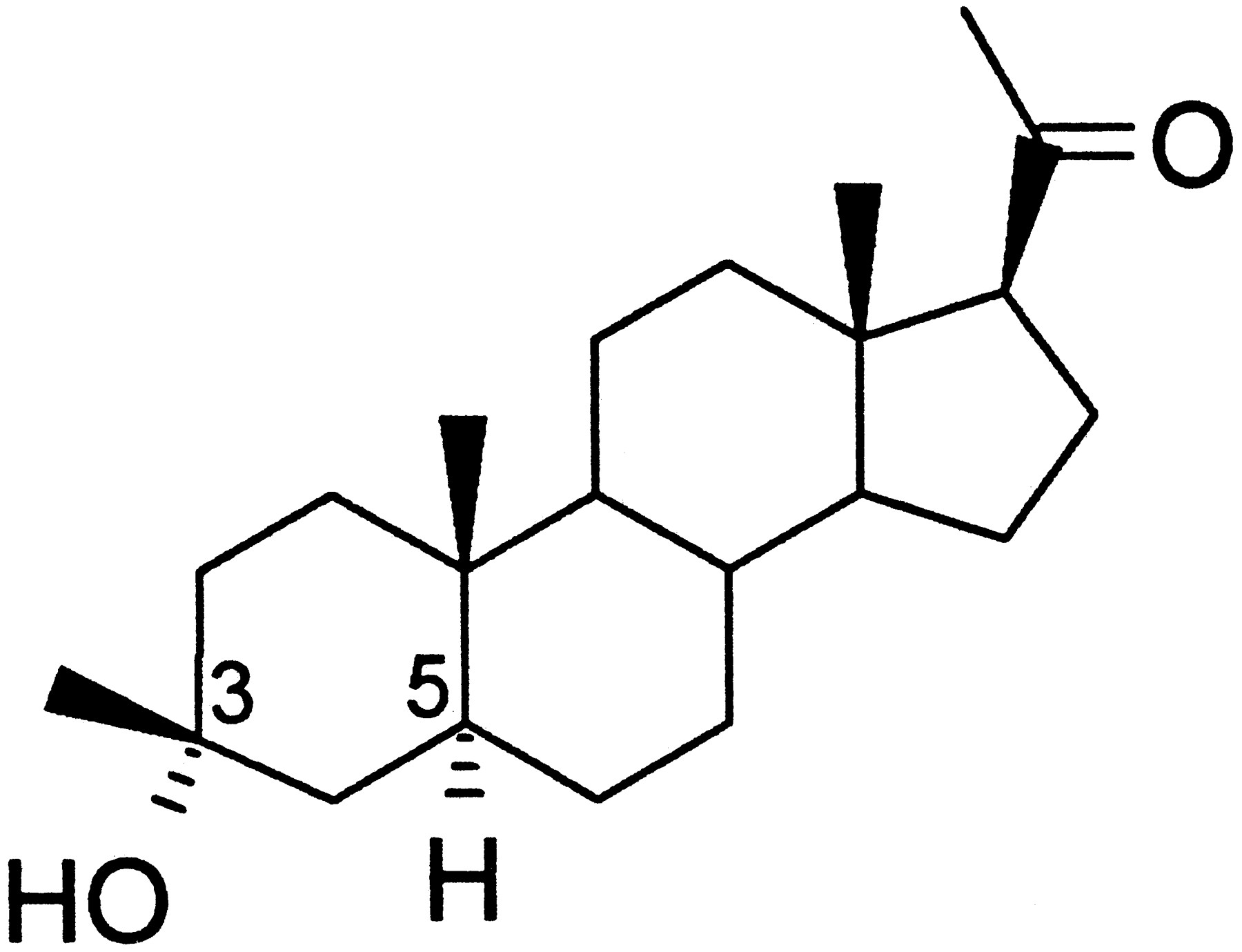
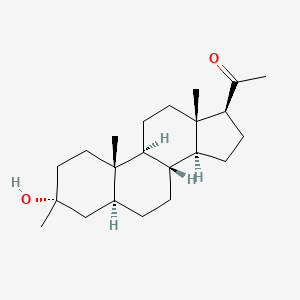
Safety and efficacy of ganaxolone in patients with CDKL5 deficiency disorder: results from the double-blind phase of a randomised, placebo- controlled, phase 3 trial - The Lancet Neurology

Marinus Pharmaceuticals Announces Delay to RAISE Phase 3 Clinical Trial in Status Epilepticus and Associated IV Ganaxolone Clinical Trials | Business Wire

Intramuscular allopregnanolone and ganaxolone in a mouse model of treatment‐resistant status epilepticus - Zolkowska - 2018 - Epilepsia - Wiley Online Library

Marinus Pharmaceuticals Provides Business Update and Reports Third Quarter 2021 Financial Results | Business Wire
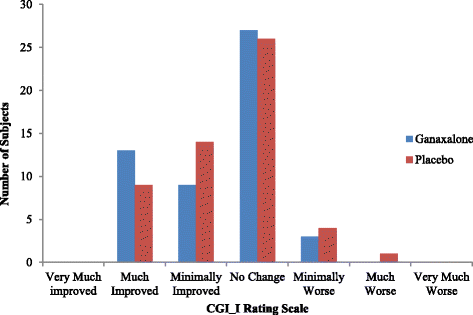
A randomized double-blind, placebo-controlled trial of ganaxolone in children and adolescents with fragile X syndrome | Journal of Neurodevelopmental Disorders | Full Text

Marinus Pharmaceuticals, Inc. Enrolls First Patient in Pivotal Phase 3 Clinical Trial of IV Ganaxolone in Refractory Status Epilepticus · BioBuzz

Randomized, double‐blind, placebo‐controlled phase 2 study of ganaxolone as add‐on therapy in adults with uncontrolled partial‐onset seizures - Sperling - 2017 - Epilepsia - Wiley Online Library