Navigating the differences between Investigator- Initiated v. Sponsor-Initiated Clinical Trials – What are the special contract and administration issues. - ppt download

1 Investigator-Initiated Clinical Research Planning, Developing, Conducting, Managing, and Succeeding Wm. Hirschhorn, M.S. Director, OCT & RQI and Adj. - ppt download

MJA on Twitter: "Value proposition of investigator‐initiated clinical trials conducted by networks … #freeaccess 1 week … "Investigator‐initiated trials run by clinical trial networks provide net economic benefits to health systems" https://t.co ...

Investigator-initiated trials of targeted oncology agents: why independent research is at risk? | Semantic Scholar
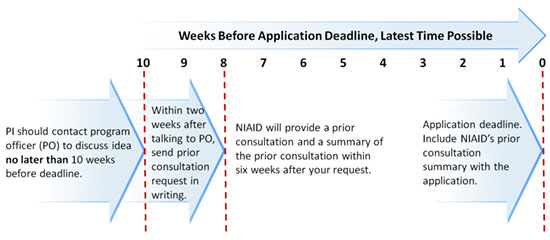
Prior Consultation Timeframes for Investigator—Initiated Clinical Trial Applications | NIH: National Institute of Allergy and Infectious Diseases